|
Collecting, Preserving and Archiving Echinoderms Gordon Hendler Natural History Museum of Los Angeles County Last updated: 12 December 2004 |
 |
1. Introduction
The Echinodermata consist of over 6,500 living species in six named classes, including asteroids, crinoids, concentricycloids, echinoids, ophiuroids, holothuroids. They are distributed from the intertidal zone to the deepest ocean trenches, often in immense numbers, and are of considerable ecological significance. Echinoderms are most diverse in the tropics, but occur in greatest profusion in productive temperate and cold-water ecosystems. The adults range in size from basket stars one meter in diameter and sea cucumbers three meters in length, to sea urchins smaller than a centimeter. Larvae and juveniles may be a millimeter or less in size. Echinoderms have a unique skeleton, which consists of numerous, highly porous, calcium carbonate ossicles (bones), each of which has the optical properties of a single calcite crystal. Moreover, echinoderms are the only animals that have “mutable connective tissue” which, together with muscles, links the ossicles and enables echinoderms to transform nearly instantaneously from a rocky rigidity to pudding-like pliancy, and just as quickly to reverse the process. Both soft tissue and skeletal characters are key to the identification and study of echinoderms. Therefore, maintaining the integrity of the skeleton and connective tissue is key to successfully preserving and archiving echinoderm specimens.
2. In situ documentation
Video Protocol: If possible, in addition to documenting the appearance of individuals in situ, video sequences should record behavior such as feeding and locomotion, and interactions with hosts and other organisms.
Transect types: Visual transects, line transects, sampling quadrats, and other systems for quantitatively documenting the abundance of large, epifaunal organisms provide a very limited indication of echinoderm diversity and abundance. A variety of sampling techniques must be combined to carry out a thorough survey because: 1) most echinoderms are cryptic, 2) echinoderms occupy a wide variety of different habitats and substrates, and 3) Adult and juvenile individuals tend to occur in different microhabitats.
3. Collecting specimens to insure scientific value
Permits: Note, it is sometimes advantageous to identify the quantity of specimens in terms of the weight, rather than the number of specimens.
Methods: When hand collecting echinoderms, for example using scuba, bear in mind that most echinoderms are cryptic, and many are nocturnal, although some live on the surface of the substrate or host organisms such as sponges, corals, and algae. Thus, a degree of "destructive sampling" is required for a thorough survey of echinoderm diversity. Individuals are found under rocks and slabs of coral rubble, which can turned over and should be restored to their original position to minimize damage to the habitat. Echinoderms can also be extracted from chunks of rock, rubble, and algal substrates that are held in containers of seawater until the medium is hypoxic, or adding a low concentration of formalin or alcohol to irritate and drive animals out of crevices. The substrate may then be broken into small pieces and stirred vigorously, the seawater decanted through a fine sieve, and the animals picked from the debris remaining at the bottom of the container. Echinoderms also emerge from rock and reef crannies in response to the ichthyotoxins (fish poisons) used by fish collectors. Burrowing brittle stars, sea cucumbers, and sea urchins are often associated with the roots of seagrasses and soft sediments. They are obtained by selectively digging, washing sediment through a sieve, or combing the sediment with one's fingers. Species can be taken from great depths by dredges, trawls, grabs, cores, or baited traps, and in excellent condition by using research submersibles and ROVs.
Most echinoderms can be manipulated with the naked hand, although the smallest individuals can be manipulated with forceps. Obviously, a degree of caution is required in handling sea urchins with sharp spines, since the spines and pedicellariae of a very few species are toxic. Delicate ophiuroids and crinoids must be manipulated gingerly to prevent autotomy, and held near the center of the body, not at the arm tips. Echinoderms should be protected from temperature shock. Individuals from cold deep water should be refrigerated, and even shallow-water, tropical species should be shielded from the sun and held in running seawater or insulated containers until they are processed.
Samples:
1] Consideration should be given to preparing duplicate samples of echinoderms, since their skeletons and soft structures are both important and must be treated differently. Specimens preserved directly in 75-85% ethanol are ideal for study of the skeleton. However, elements of the skeleton degrade in the fixatives (even buffered formaldehyde) used for the fixation of soft tissue.
2] An effort should be made to preserve and archive a range of sizes of each species collected. Echinoderm morphology changes markedly as individuals grow, and having a size-series of specimens can make it possible to identify problematical small and juvenile specimens. Information on the size-range and a representative sample of the sizes of individuals in a populations provides useful ecological information as well.
Subsamples: Subsamples of specimens intended for DNA or RNA sequencing typically consist of an arm or a portion of an arm of asteroids, ophiuroids and crinoids, and a portion of the body wall of holothuroids. Tube feet and gonadal tissue are also harvested.
Photography: Color records of echinoderms can facilitate identification and provide valuable information on diagnostic pigmentation and on the intraspecific variability of pigmentation and color patterns. With regard to accurate identification, photographs are not a substitute for a preserved specimen, but they augment the value of preserved, decolorized specimens.
Orientation, views: In general, photographic images should include overall dorsal and ventral views of individuals, and details of the dorsal and ventral surface of individuals that have been anesthetized and have tentacles and arm extended. Echinoderms can be successfully photographed by holding a camera held above the subject that is resting in a glass-bottomed container, which is supported above a background such as black velvet or neutral gray. The container is partially filled with seawater or a “relaxing” medium such as magnesium chloride or Epsom salts solution. Underwater photographs are of value for information they convey about appearance, behavior and ecology of echinoderms.
Metadata: Locality (as precisely as possible, with coordinates of latitude and longitude if available), depth, date, name of collector, and if possible collection gear, preservative, vessel (e.g. name of dive boat), and notes on habitat, microhabitat, and associations. It is important to record color of living echinoderms if a photographic record is not made.
Collecting effort: Data from semi-quantitative collections are useful if collecting methodology is standardized, for example, collecting all individuals detected or the largest and smallest individuals found in a given period of time.
Habitat: Microhabitat and associations with particular substrates or host species should be noted.
4. Preserving specimens to enhance long-term investigation and study
Dried Museum Specimens: Dried museum specimens are prepared to facilitate the examination of skeletal structures for taxonomic study. They are also used when storage in alcohol is not possible, as when alcohol is unavailable in insufficient in quantity to preserve large specimens. However, alcoholic specimens are more versatile, and generally preferable if the level of alcohol concentration is monitored, and maintained at 75-85 %. Specimens to be dried
Tissue Fixation: Typically, specimens are fixed if they are to be used for the gross anatomical study of soft tissue structure, or for histology. Buffered formaldehyde is a general-purpose fixative for echinoderms, and the fixatives used for SEM samples are also used for histology. Acidic buffers are useful for material intended for sectioning because they dissolve the calcareous skeleton. Release of gas by the dissolving skeleton, however, can damage fine tissue microstructure, and when that is a consideration, tissue is treated with chelating agents such as EDTA or dilute acidic solution after fixation.
Tissue Preservation: Echinoderm tissue is best preserved in 75-85% ethanol. Alcohol concentration must be maintained, and echinoderm collections should not simply be “topped off” without monitoring the alcohol concentration. Dilute alcohol solutions tend to become acidic, which leads to the decalcification of skeletal structures and the deterioration of soft tissue, and renders specimens useless for study and worthless as vouchers.
SEM Analysis: Typically echinoderm tissue samples and larvae are fixed in a 2% solution of glutaraldehyde in seawater or a buffer, which may be followed by postfixation in osmium tetroxide. Bouin’s and buffered 5% formaldehyde are also used successfully. The appropriate fixative depends on the precise structures and tissue under study.
DNA analysis: DNA has successfully been extracted from tissue stored in 95% ethanol, or for shipment, in a solution DMSO and EDTA saturated with NaCl.
Basic Preservation Procedure for Echinoderms
Place the specimens in a tightly sealed container of 70% alcohol. When undenatured ethanol is unavailable, vodka, rum, or isopropanol (rubbing alcohol) can be substituted. If alcohol is in short supply, large sea cucumbers can be eviscerated before preservation by making an incision in the body, removing the internal organs, and preserving only the body wall. Formalin (preferably 5-10% formalin buffered with sodium borate), can be used as a preservative for most echinoderms, but sea cucumbers should always be stored in alcohol. Formalin is only suitable for short-term storage, since it is inherently acidic, and prolonged contact destroys the echinoderm skeleton and makes specimens brittle. The quality of specimens can be improved by relaxing specimens and handling them as described in the following section.
Optimal Preservation Procedure for Echinoderms
Museum-quality specimens are prepared by following the five following steps, but for specialized applications such as histological or biochemical studies different techniques may be required.
(1) Cleaning. Animals that are covered with sediment or debris should be gently agitated or rinsed with a stream of seawater.
(2) Relaxation. Relaxation (anesthesia) prevents specimens from breaking or contorting when they are preserved. Echinoderms are anesthetized in covered trays, which exclude light, by soaking them in isotonic magnesium chloride or magnesium sulfate solutions. The effect of these chemicals is reversible; individuals recover from anesthesia when returned to seawater. An alternative method for tropical echinoderms involves placing animals in a container of seawater that is at room temperature, and leaving them overnight in a refrigerator. In that time, they die in a "relaxed" posture, but delicate tissue structures may be damaged. Special relaxing methods for sea cucumbers, large sea stars, feather stars and basket stars are explained below.
Relaxation with magnesium chloride is a preferred treatment for specimens to be used for histological analysis, or for scanning electron microscopy of soft tissues, and is ideal for many echinoderms. Individuals are transferred from seawater, directly into a container with a solution of magnesium chloride that is isotonic with seawater. Anesthesia is usually complete in 15 minutes.
It can be more convenient to use magnesium sulfate (epsom salts), since it is available from any pharmacy, and need not be weighed before use. Place specimens in a shallow container (a tray) and barely cover them with seawater. Put 1-3 tablespoons of epsom salts in a corner of the container (not directly on the specimens). Every five minutes, gently tip the tray to mix the dissolving salts. If the specimens actively move after 20 minutes, sparingly add more salts (the amount of salts required is proportional to the volume of water in the tray).
Many sea cucumbers respond rapidly to magnesium chloride and magnesium sulfate, but some will not even relax after hours in magnesium salts. For refractory species, propylene phenoxytol (PPO) may be used. Place a few milliliters of concentrated PPO (this chemical must be handled with caution) in a small bottle of fresh water. Shake the bottle vigorously, then let the tiny drops of PPO settle to the bottom. Draw off a pipette (medicine dropper) of the supernatant liquid and add it to a container of seawater with the sea cucumber; add additional pipettes full of the mixture at 1-2 hour intervals until the specimen extends its feeding tentacles. Leave the specimen in the solution until it is completely unreactive to prodding - overnight if necessary.
Large seastars, which would require an inordinate amount of magnesium salts for anesthetization, can be relaxed using freshwater, if they are not to be used for the study of soft tissue structure. Place specimens in a bucket or a tray with just enough seawater to cover them, and wait for them to extend their arms. At intervals of about 20-30 minutes, add freshwater to the container to dilute the seawater from full strength to 90%, 80%, 70%, and so on. Usually it is not necessary to dilute the seawater to less than 50% for thorough relaxation.
Feather stars and basket stars do not respond to standard anesthetics such as magnesium salts, but some species of feather stars relax after 10 minutes in calcium-free seawater followed by 10 minutes in approximately 0.1% MS-222 (a fish anesthetic). However, feather stars and basket stars can be preserved without relaxation, as described in the following section on preservation.
It is not necessary to relax sea urchins before preservation. Note, that if an urchin with long, outspread spines does not fit in a narrow container, its spines may be induced to "fold up" if the live individual is balanced on the opening of the seawater-filled container, and allowed to settle to the bottom.
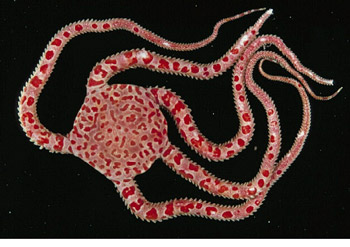
(3) Preservation. Relaxation is complete when the tube feet, arms, or tentacles of an individual do not react to prodding; at that point, the specimen is placed in preservative. In general, the best preservative for echinoderm specimens is 70% ethanol, but formalin can sometimes be substituted, as specified below. Since specimens stiffen in preservative, relaxed sea stars, brittle stars, and sea cucumbers should be carefully stretched out in a tray of alcohol to harden them in a suitable position for study. To facilitate storage, a brittlestar's disk and arms can be arranged in a U-shape, or "comet" form with arms flat and trailing behind the disc (see image at top of document). Pick up the disk of a relaxed brittle star between two fingers (or with forceps); gently shake the specimen so that the arms straighten, before placing it in alcohol.
The tentacles of completely relaxed sea cucumbers will not contract when individuals are transferred to alcohol. Very large individuals, or animals with a thick body wall, should be preserved using alcohol injection. While the completely relaxed specimen is still in PPO or magnesium chloride solution, 95% alcohol is carefully injected through the anus of the sea cucumber with a hypodermic syringe. When the tentacles are fully extended, the anus is pressed closed for several minutes, before transferring the specimen to 70% alcohol. If anesthetic is unavailable, a sea cucumber with extended tentacles can be quickly, tightly grasped with tweezers behind its tentacles and held in alcohol for several minutes before releasing it.
Feather stars and basket stars, which have not been relaxed, are preserved as follows. Keep an individual in a darkened container of seawater until it extends its arms (nocturnal species may not extend their arms until the night). Fill a tray with ethanol, preferably a 90-95% concentration. Lift the individual specimen out of seawater, supporting its arms so that they do not cover the central region of the body. Rapidly transfer it to the tray of alcohol, and maintain just enough gentle pressure on the specimen to keep the arms extended for a minute or two, until the specimen stiffens. Sea urchins can be transferred from seawater to a wide mouthed bottle of alcohol without any relaxation treatment.
Most echinoderms can be preserved in 5-10% buffered formalin. However, sea cucumbers must be preserved in alcohol because their microscopic ossicles, which are taxonomically diagnostic, quickly dissolve in mildly acidic solutions. Small or delicate echinoderms left in formalin for long periods will deteriorate; they should be transferred to alcohol within several days. Initial preservation in formalin may be beneficial for large specimens of starfish and sea urchins. However, they should not be stored in formalin for more than a few weeks before being transferred to alcohol or dried. In the field, if many specimens are to be processed in formalin, the solution may be re-used.
(4) Washing and Drying. The preservative solution in which specimens are first placed invariably becomes diluted, acidified, and contaminated with seawater salts or formalin, and eventually damages wet-preserved or dried material. Therefore, before drying or transfer to fresh alcohol, large formalin-fixed specimens should be soaked overnight in distilled water to remove formalin and salts. After washing in distilled water, drain specimens and place them in clean, 70% ethanol. Large specimens (or large numbers of specimens) should be transferred through two changes of alcohol after they are washed, because the wash water they release dilutes the first preservative bath.
Alcohol preservation is the most satisfactory method for long-term storage of echinoderms because it inhibits the deterioration of the skeleton and soft tissue. However, echinoderm specimens may be dried for several reasons. Some must be examined dried, or are too large to store in alcohol containers. Also, formalin-preserved and dried specimens may retain their natural coloration for extended periods if they are stored in the dark. A specimen that had been preserved in formalin can be dried after it is washed in water; specimens preserved in alcohol need not be washed. The specimen is placed on a support, such as a metal screen, in a warm, dry well-aerated place (e.g., under a lamp but not in direct sunlight or an oven), until it is completely desiccated.
(5) Storage. Preserved specimens may be stored in plastic or glass containers. For temporary storage, plastic bags, such as whirl-paks or zip-locks are useful for small specimens; animals with sharp spines can be kept in plastic bottles or metal paint cans. Before shipping, samples may be drained, leaving moistened specimens in a minimal volume of preservative. For long-term storage it is best to keep echinoderms in glass containers with tightly sealed plastic lids, in a volume of 75-85% ethanol at least twice their body volume.
Never cushion wet or dried echinoderms with cotton of other fibrous materials. The threads become tangled in spines and other delicate structures and damage specimens. Cotton plugs in shell vials with small specimens should be enclosed in a layer of lens paper or another tissue.
5. Labelling: Be aware that some label papers are acidic. Over time, delicate specimens enclosed in a container with large amount of label paper can be decalcified, and rendered useless for study.
6. Shipping: Never use cotton or other fibrous material as padding for echinoderm specimens (see above).
References
Clark, Ailsa M. 1977. Starfishes and Related Echinoderms. Neptune City, New Jersey: T.F.H. Publications.
Harrison, Frederick W. and Fu-Shiang Chia, eds. 1994. Microscopic Anatomy of Invertebrates. Volume 14. Echinodermata. New York: Wiley-Liss.
Hendler, Gordon, John E. Miller, David L. Pawson and Porter M. Kier. 1995. Sea Stars, Sea Urchins, and Allies: Echinoderms of Florida and the Caribbean. Washington: Smithsonian Institution Press.
Hyman, Libbie H. 1955. The Invertebrates: Echinodermata. The Coelomate Bilateria. New York: McGraw-Hill.
Lawrence, John 1987. A Functional Biology of Echinoderms. London: Croon Helm.
Giese, Arthur C., John S. Pearse, and Vicki B. Pearse. 1991. Reproduction of Marine Invertebrates. Volume VI. Echinoderms and Lophophorates. Pacific Grove, California: The Boxwood Press.
|
|
||||